Emma-Ruoqi Xu, Aleix Lafita, Alex Bateman, Marko Hyvönen
Acta Crystallographica Section D Structural Biology D76:124-134 (2020)
DOI: 10.1107/S2059798319016747
Pubmed: 32038043
PDB coordinates:
Abstract
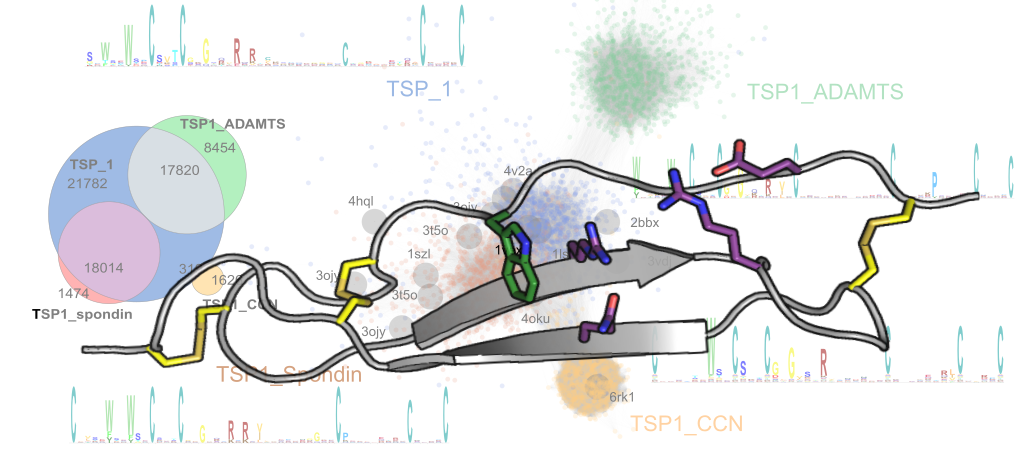
The members of the CCN (Cyr61/CTGF/Nov) family are a group of matricellular regulatory proteins that are essential to a wide range of functional pathways in cell signalling. Through interacting with extracellular matrix components and growth factors via one of their four domains, the CCN proteins are involved in critical biological processes such as angiogenesis, cell proliferation, bone development, fibrogenesis and tumorigenesis. Here, the of the thrombospondin module 1 (TSP1) domain of CCN3 (previously known as Nov) is presented, which shares a similar three-stranded fold with the thrombospondin type 1 repeats of thrombospondin-1 and spondin-1, but with variations in the disulfide connectivity. Moreover, the CCN3 TSP1 domain lacks the typical π-stacked ladder of charged and aromatic residues on one side of the domain that is seen in other TSP1 domains. Using conservation analysis among orthologous domains, it is shown that a charged cluster in the centre of the domain is the most conserved site and this cluster is predicted to be a potential functional for heparan sulfate binding. This variant TSP1 domain has also been used to revise the sequence determinants of TSP1 domains and to derive improved Pfam sequence profiles for the identification of novel TSP1 domains in more than 10 000 proteins across diverse phyla.