Katrin Fischer, Aleksei Lulla, Tsz Y So, Pehuén Pereyra-Gerber, Matthew I. J. Raybould, Timo N. Kohler, Tomasz S. Kaminski, Juan Carlos Yam-Puc, Robert Hughes, Florian Leiß-Maier, Paul Brear, Nicholas J. Matheson,Charlotte M. Deane, Marko Hyvönen, James E. D. Thaventhiran, Florian Hollfelder
BioRxiv, posted 12 Jan 2023
DOI: 10.1101/2023.01.10.523494
PDB coordinates: 8BE1 (3D view)
Plasmids in Addgene: pExp-His-ZBasic-RBD, pExp-His-ZBasic-RBD-Avi
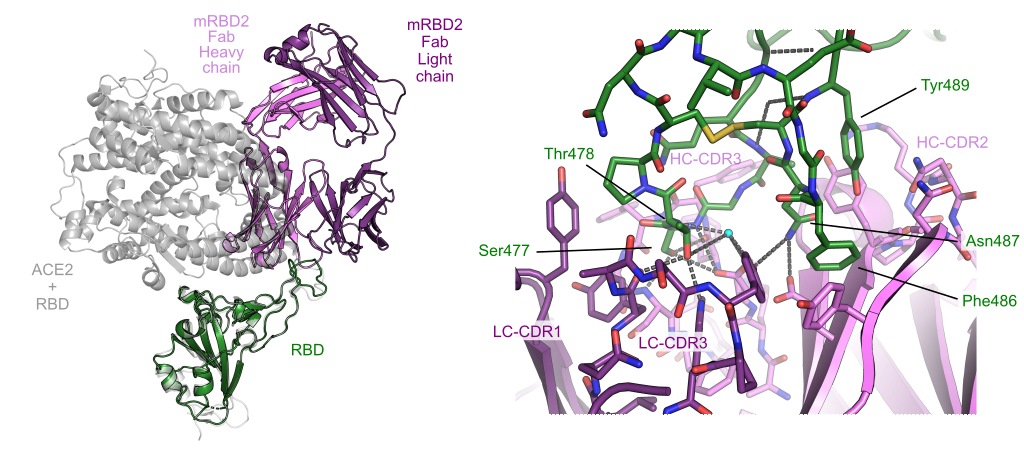
Abstract
Monoclonal antibodies are increasingly used to prevent and treat viral infections, playing a pivotal role in pandemic response efforts. Antibody secreting cells (ASCs, plasma cells and plasmablasts) are an excellent source of high-affinity antibodies with therapeutic potential. Current methodologies to study antigen-specific ASCs either have low throughput, require expensive and labour-intensive screening or are technically demanding and therefore not accessible to the wider research community. Here, we present a straightforward technology for the rapid discovery of monoclonal antibodies from ASCs: we combine microfluidic encapsulation of single cells into an antibody capture hydrogel with antigen bait sorting by conventional flow cytometry. With our technology, we screened millions of mouse and human ASCs and obtained anti-SARS-CoV-2 monoclonal antibodies with high affinity (pM) and neutralising capacity (<100 ng/mL) in two weeks with a high hit rate (>85%). By facilitating access into the underexplored ASC compartment, we enable fast and efficient antibody discovery as well as immunological studies into the generation of protective antibodies.
