2015
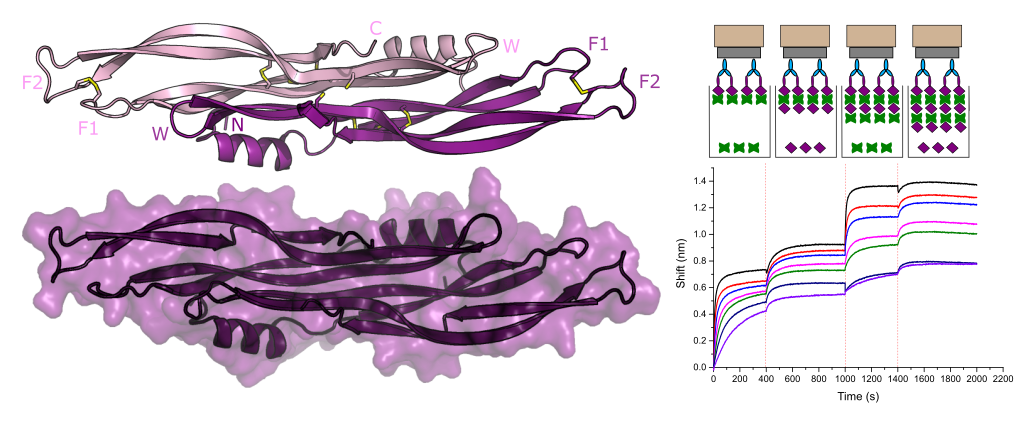
Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics.
P.-Y. Colin, B. Kintses, F. Gielen, C. M. Miton, G. Fischer, M. F. Mohamed, M. Hyvönen, D. P. Morgavi, D. B. Janssen, F. Hollfelder
Nature Communications. 6:10008 doi: 10.1038/ncomms10008, 2015.
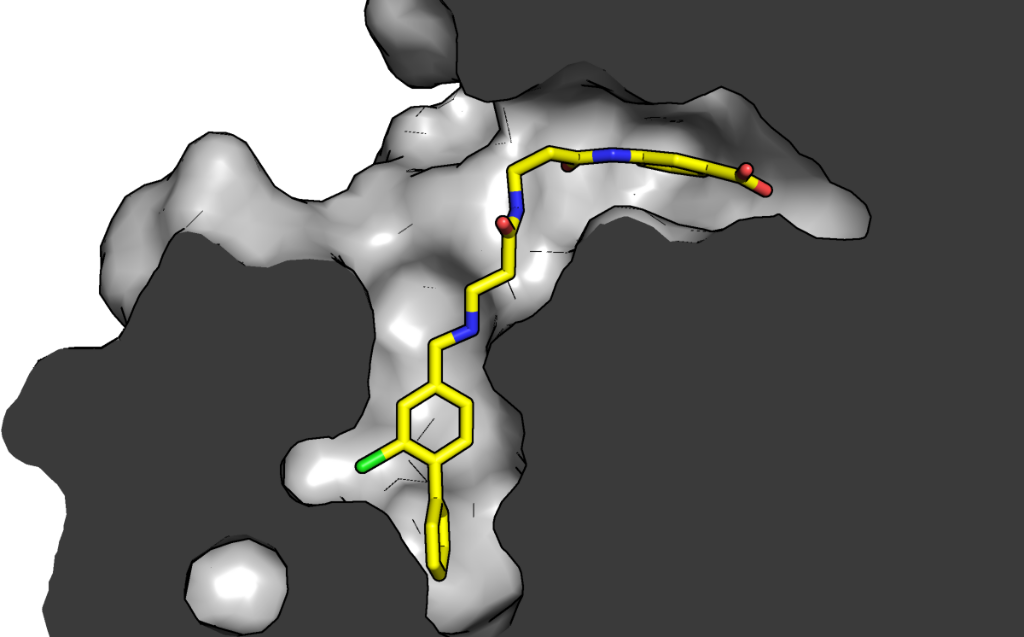
Alternative Modulation of Protein-Protein Interactions by Small Molecules.
G. Fischer, M. Rossmann, M. Hyvönen
Current Opinion in Biotechnology, 35:78-85 doi: 10.1016/j.copbio.2015.04.006, 2015.
2014
Small molecule inhibitors targeting protein-protein interaction in the RAD51 family of recombinases
D.E. Scott, A. G. Coyne, T. L. Blundell, A. Venkitaraman, C. Abell, M. Hyvönen
ChemMedChem, 10: 296–303 DOI: 10.1002/cmdc.201402428, 2014
Functionalised staple linkages for modulating the cellular activity of stapled peptides.
Y. H. Lau, P. de Andrade, S.-T. Quah, M. Rossmann, L. Laraia, N. Sköld, T. J. Sum, P. J. E. Rowling, T. L. Joseph, C. Verma, M.Hyvönen, L. S. Itzhaki, A. R. Venkitaraman, C. J. Brown, D. P. Lane, D. R. Spring
Chemical Science, 5:18040-1809, doi: 0.1039/C4SC00045E, 2014
2012
Targeting the RAD51:BRCA2 Protein-Protein Interaction using Fragment-based methods.
D. E. Scott, M. T. Ehebauer, T. Pukala, M. Marsh, T. L. Blundell, A. R. Venkitaraman, C. Abell, M. Hyvönen. ChemBioChem,14:332-42, 2013
A. Sharma, F. Meyer, M.Hyvönen, S. M. Best, R. E. Cameron, N. Rushton. Osteoinduction by combining bone morphogenetic protein (BMP)-2 with a bioactive novel nanocomposite
Bone and Joint Research. 1:145-51, 2012
2011
Targeting protein-protein interactions and fragment-based drug discovery.
E. Valkov, T. Sharpe, M. Marsh, S. Greive and M. Hyvönen
In Topics in Current Chemistry: “Fragment-based drig discovery and X-ray crystallography” ed. T. Davies and M. Hyvönen, 2011, DOI: 10.1007/128_2011_265
From crystal packing to molecular recognition: prediction and discovery of a binding site on the surface of polo-like kinase 1.
P Sledź, C. J. Stubbs, S. Lang, Y. Q. Yang, G. J. McKenzie, A. R. Venkitaraman, M. Hyvönen, C Abell
Angewante Chemie International Edition, 50:4003-6, 2011
2010
An efficient,multiply promiscuous hydrolase in the alkaline phosphatase superfamily.
B. van Loo, S. Jonas, A. C. Babtie, A. Benjdia, O. Berteau, M. Hyvönen, and F. Hollfelder
Proceedings of the National Academy of Sciences of the USA, 107:2740-5, 2010
2009
Rab5-mediated endocytosis of activin is not required for gene activation or long-range signalling in Xenopus.
A. I. Hagemann, X. Xu, O. Nentwich, M. Hyvönen, K. Dingwell and J. C. Smith
Development, 136:2803-13, 2009
A new ‘total’ activin B ELISA: development and validation for human samples.
H. Ludlow, D. J. Phillips, M. Myers, R. I. McLachlan, D. M. de Kretser, C. A. Allan, R. A. Anderson, N. P. Groome, M. Hyvönen, W. C. Duncan and S. Muttukrishna
Clinical Endocrinology. 71:867-73, 2009
2008
A new member of the alkaline phosphatase superfamily with a formylglycine nucleophile: structural and kinetic characterisation of a phosphonate monoester hydrolase/phosphodiesterase from Rhizobium leguminosarum.
S. Jonas, B. van Loo, M. Hyvönen, F. Hollfelder
Journal of Molecular Biology, 384:120-36, 2008
Development of a new antibody to the human inhibin/activin βB subunit and its application to improved inhibin B ELISAs.
H. Ludlow, S. Muttukrishna, M. Hyvönen , N.P. Groome
Journal of Immunological Methods, 329:102-111, 2008
2006
Structural basis for the inhibition of activin signalling by follistatin.
A.E. Harrington, S.A. Morris-Triggs, B.T. Ruotolo, C.V. Robinson, S. Ohnuma and M. Hyvönen
EMBO Journal, 25:1035-1045, 2006
2003
CHRD, a novel domain in the bone morphogenetic protein inhibitor chordin, is also found in microbial proteins.
M. Hyvönen
Trends in Biochemical Sciences, 28: 470–473, 2003
Adhesion of endothelial cells to nov is mediated by integrins αv3 and β51.
P. D. Ellis, J. C. Metcalf, M. Hyvönen, and P. R. Kemp
Journal of Vascular Research, 40:234–243, 2003
Crystal structures of the heparan sulfate-binding domain of follistatin. Insights into ligand binding.
C. A. Innis and M. Hyvönen
Journal of Biological Chemistry, 278:39969–39977, 2003
2000
Protein-protein interactions in eukaryotic signal transduction.
M. Hyvönen, J. Begun, and T. Blundell
In Protein-Protein Recognition, Frontiers in Molecular Biology, pages 189–227. Oxford University Press, 2000
1999
Structure of the PH domain from Bruton’s tyrosine kinase in complex with inositol 1,3,4,5-tetrakisphosphate.
E. Baraldi, K. Djinovic-Carugo, M. Hyvönen, P. Lo Surdo, A. M. Riley, B. V. L. Potter, R. O’Brien, J. E. Ladbury, and M. Saraste
Structure, 7:449–460, 1999
Expression of cDNAs in Escherichia coli using T7 promoter.
M. Hyvönen and M. Saraste.
In Cell Biology – A laboratory manual, volume 4, pages 255–261. Academic Press, 2nd edition, 1997a
Structure of PH domain and Btk motif from Bruton’s tyrosine kinase: molecular explanations for the X-linked agammaglobulinemia.
M. Hyvönen and M. Saraste
EMBO Journal, 16:3396–3404, 1997
Structure of the WW domain of a kinase-associated protein complexed with a proline-rich peptide.
J. Macias, M. Hyvönen, E. Baraldi, J. Schultz, M. Sudol, M. Saraste, and H. OschkinatNature, 382:646–649, 1996.
T7 vectors with modified T7lac promoter for expression of proteins in Escherichia coli.
J. Peränen, M. Rikkonen, M. Hyvönen, and L. Kääriäinen
Analytical Biochemistry, 236:371–373, 1996.
The alphavirus replicase protein nsP1 is membrane-associated and has affinity to endocytic organelles.
J. Peränen, P. Laakkonen, M. Hyvönen, and L. Kääriäinen
Virology, 208:610–620, 1995.
Structure of the binding site for inositol phosphates in a PH domain.
M. Hyvönen, M. J. Macias, M. Nilges, H. Oschkinat, M. Saraste, and M. Wilmanns
EMBO Journal, 14:4676–4685, 1995.
Pleckstrin homology domains: a fact file.
M. Saraste and M. Hyvönen
Current Opinion in Structural Bioloy, 5:403–408, 1995.
The C-terminal domain of α-spectrin is structurally related to calmodulin.
G. Trave, A. Pastore, M. Hyvönen, and M. Saraste
European Journal of Biochemistry, 227:35–42, 1995.
PH domain: the first anniversary.
T.J. Gibson, M. Hyvönen, A. Musacchio, M. Saraste, and E. Birney
Trends in Biochemical Sciences, 19:349–353, 1994.
Expression of Semliki forest virus nsP1-specific methyltransferase in insect cells and in Escherichia coli.
P. Laakkonen, M. Hyvönen, J. Peränen, and L. Kääriäinen
Journal of Virology, 68:7418–7425, 1994.